Electronic Data Capture System
An electronic data capture (EDC) system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials.Our electronic data capture (EDC) system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials by our international client.
The EDC desigend by Quantum Analytics replaces the traditional paper-based data collection methodology to streamline data collection and expedite completion of complex researches/clinical trials.
Typically, EDC systems provide:
- a graphical user interface component for data entry
- a validation component to check user data
- a reporting tool for analysis of the collected data
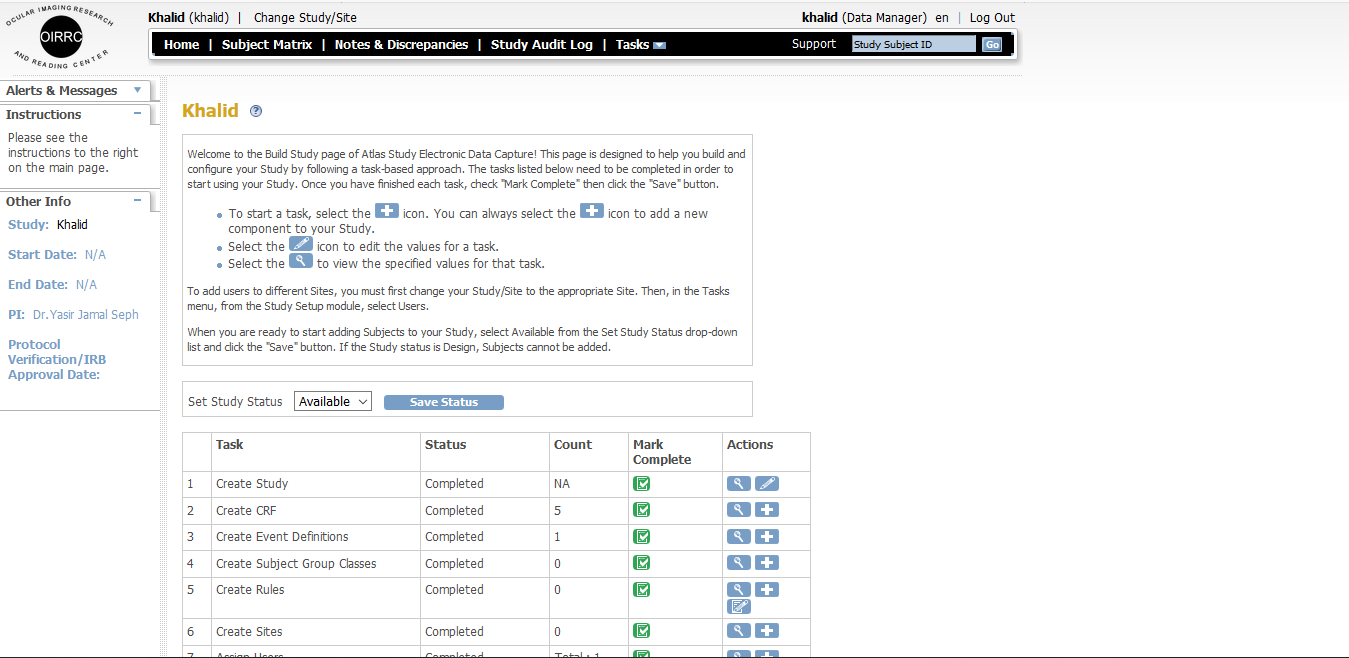
1.1 Atlas Study
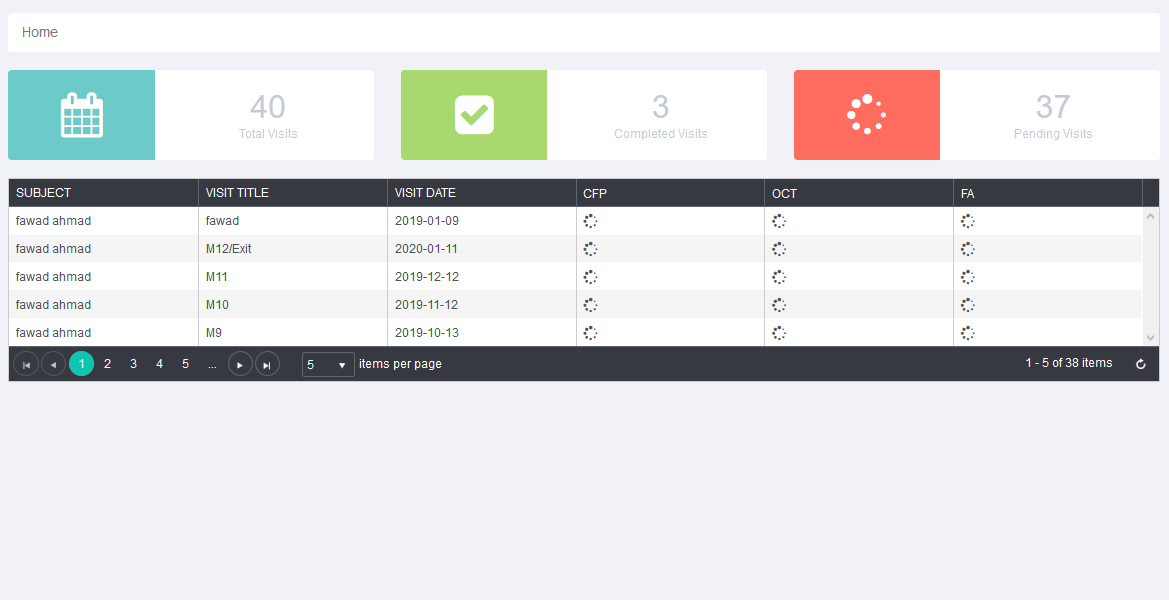
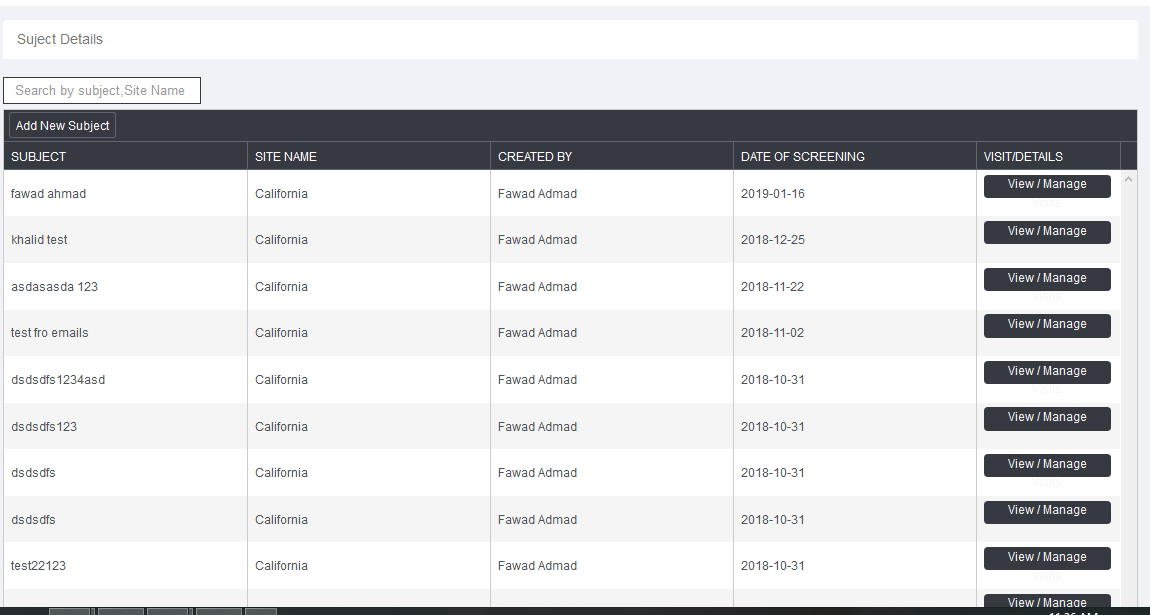
The Atlas study has been facilitated with online Case Report Form system which is utilized for data collection in clinical trials. It contains multiple modules and features. Study CRFs have been configured by OUR team – making it a ready-to-go system, which is used to collect data and management of the project.
it has following features.
- Online CRF are used to manage multiple projects / studies at same time.
- Online CRF system fit almost all projects and anytime of interventional or post marketing study can be conducted using this tool.
- We can customize our EDC system to your specific project at any given stage according to specific protocol and down the system validates it in accordance with data validation plan.
- We consider efficiency from the client’s perspective.
- Feel free to provide us with study protocol and paper CRF and you will get a fully configured and validate system that is online CRF of flexible validate efficiency
1.2 Lumina Study
Our Global clients uses this system through its scientists located in different geographical locations. It’s a flexible system facilitating electronic data capture system keeping the specific clients’ needs in view.
Main features.
- This system can manage events and can host different studies at the same time.
- Engage study participants on their own devices to collect cleaner data faster, with user- friendly forms
- It manage multiple users with different role, rights and privileges. There are different types of form managed by different users at different stages.
- Multiple check and conditions have been used
- A comprehensive activity log has been provided.
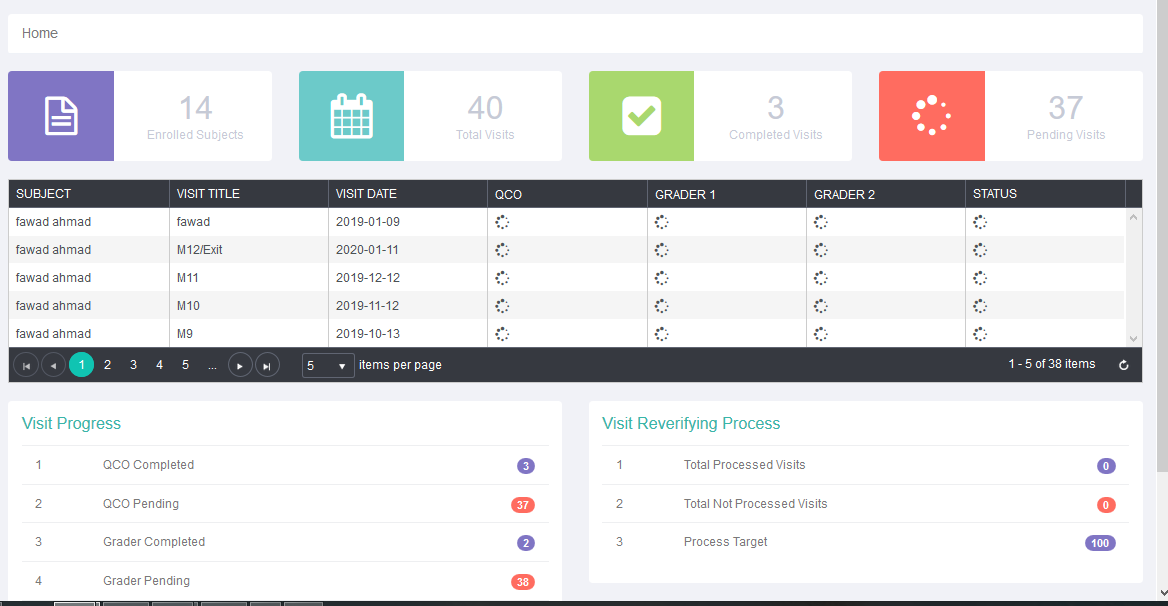
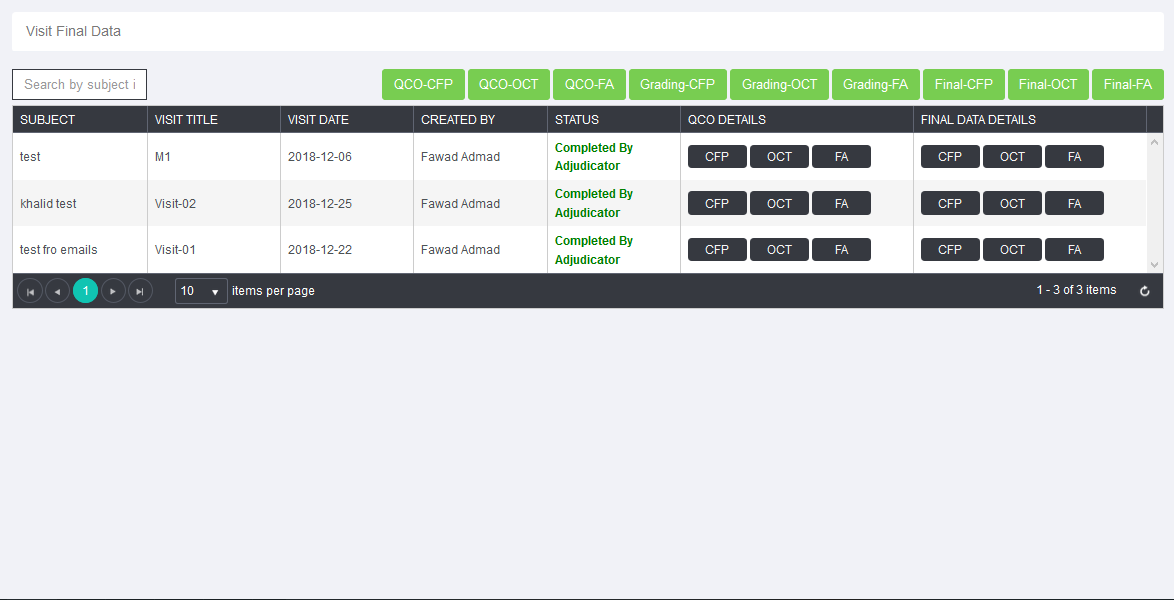
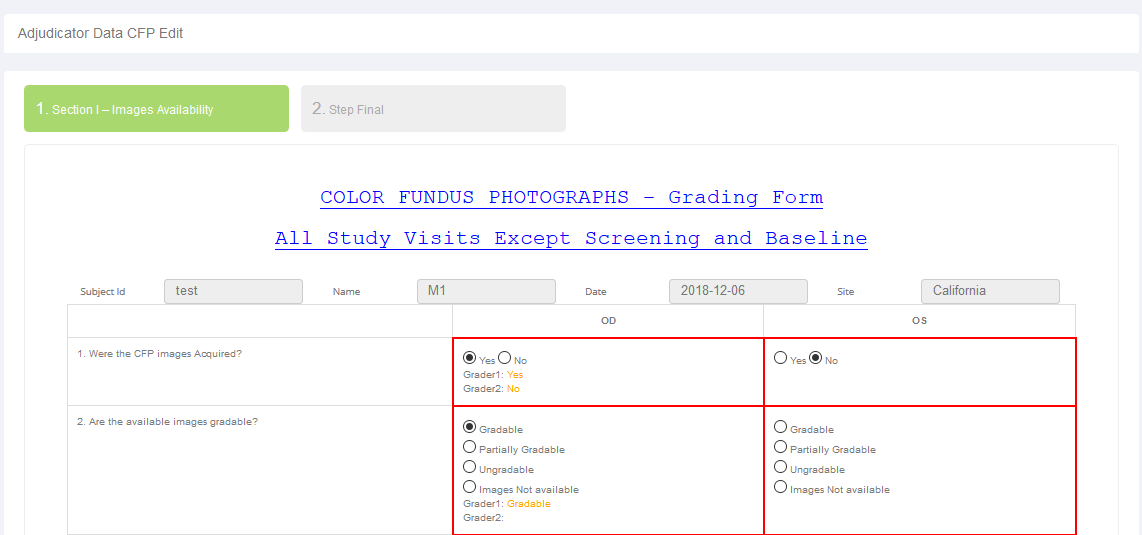
- Management of the site, data entry, data comparison of two graders and adjudication in case of variation facilitated.
- Data download in different format facilitated for processing by end user
- Guest only seen the subjects visits summary, subjects grading status table and visit progress table.